Manufacturing & Quality>Production Realization

Production Realization
In planning product realization, HCH Group will determine the following, as appropriate:
● Quality objectives and requirements for the product.
● The need to establish processes, documents, and provide resources specific to the product.
● Required verification, validation, monitoring, inspection and test activities specific to the product and the criteria for product acceptance.
● Records needed to provide evidence that the realization processes and resulting product could meet customer requirements and references to its technical specifications shall be included in the planning of product realization as a component of the quality plan.
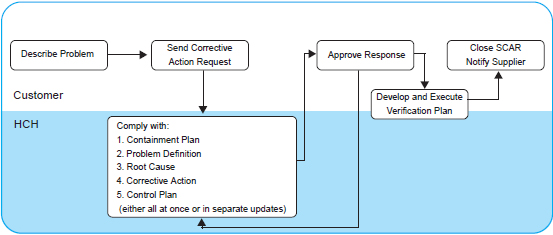
● Corrective Action Request
This procedure applies to quality failures and delivery issues with production parts and raw materials detected at HCH’s Customer.
● Quality objectives and requirements for the product.
● The need to establish processes, documents, and provide resources specific to the product.
● Required verification, validation, monitoring, inspection and test activities specific to the product and the criteria for product acceptance.
● Records needed to provide evidence that the realization processes and resulting product could meet customer requirements and references to its technical specifications shall be included in the planning of product realization as a component of the quality plan.
| Acceptance criteria | HCH Group will define the acceptance criteria and, where required, approved by the customer. For attribute data sampling, the acceptance level shall be zero defects. |
| Change control | HCH has to set up a process to control and react to changes that impact product realization. Changes shall be validated before implementation. |
| Zero defect products | In theory, this is the description of an ideal state. In reality though, we are constantly confronted with different kinds of disturbances that dramatically increase the risk of defects in manufactured products. |
| Traceability | Where traceability is a requirement, we control and record the unique identification of our products. HCH has retain manufacturing records for a minimum of 3 years, unless otherwise ?specified, |
| Preventive action | HCH Group shall determine action to eliminate the causes of potential nonconformities in order to prevent their occurrence. Preventive actions shall be appropriate to the effects of the potential problems. |
| Problem solving | HCH Group shall have a defined process for problem solving leading to root cause identification and elimination. Records of these analyses shall be kept and made available upon request. |
| Corrective action | HCH Group shall take action to eliminate the cause of nonconformities in order to prevent recurrence. Corrective actions shall be appropriate to the effects of the nonconformities ?encountered. |
● Corrective Action Request
This procedure applies to quality failures and delivery issues with production parts and raw materials detected at HCH’s Customer.





